Polyurethane
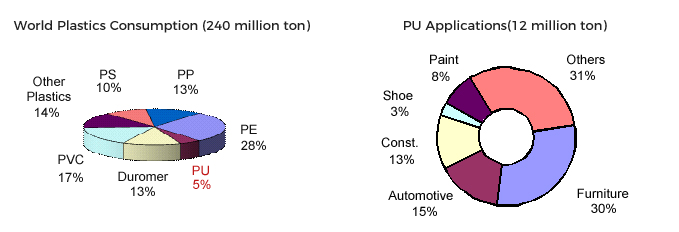
Invention of polyurethane took place in 1937 by coincidence, while conducting synthetic fibre studies in Bayer laboratories. Due to second World War, meeting with polyurethane in our daily life delayed some 10-15 years. Since the structure was flexible and blowing CO2 was reducing the final density, extensive application field was found in mattress production. It is still the main application in PU industry. After flexible foam, rigid foam is the second biggest application with various fields. Rigid foams with closed cells, similar to honeycomb structure, has a thermal insulation property. The most extensive applications of this class are counted as refrigeration and metal panel industries. Moreover polyurethanes are widely used in other industries as automotive, footwear, adhesives, cast elastomers, electrical insulation resins and paints.Main polyurethane components, polyols and isocyanates, are being produced by a various and complicated processes of Naphta which is a basic output of crude oil. As polyurethanes could be generally classified as flexible and rigid; another approach could divide them as cellular and solid (amorph) as well.
Generally polyols are classified as poly-eter and poly-ester polyols whereas isocyanates are divided as Toluene Di Isocyanate (TDI) and diphenyl Methane Di Isocyanate (MDI). It could also be mentioned about Naphtalene Di Isocyanate (NDI) which is used in small proportion in high performance elastomers.
E-Newsletter



